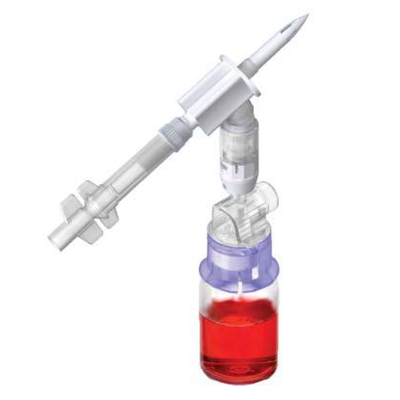
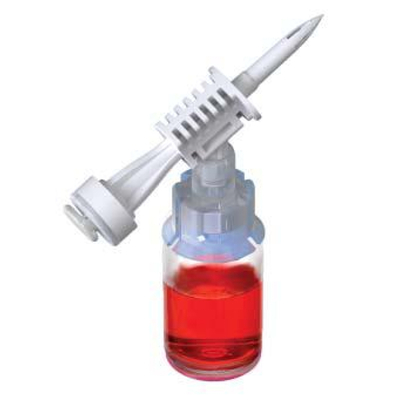
ProSeal™ Add Mixture Device (ADRx)
All Round Medication Transfer
ADRx Devices simplify the transfer of various medications, including powdered (lyophilized agents) and liquid drugs, from vials to flexible bags, eliminating the need for syringes and needles.
Effortless Process
By connecting the flexible bag to the vial using the ProSeal™ ADRX device, inverting it, and gently squeezing the bag, the vial's pressure increases as air is introduced. Releasing the pressure on the bag allows medication from the vial to be transferred seamlessly into the final container. This allows healthcare professionals and patients to experience a smooth medication transfer.
Versatility and Ease of Use
ProSeal™ ADRx Devices set new standards in versatility and ease of use, allowing medication transfer into any medication bag through both outlet and inlet ports.
COMPLIANCE
INTERNATIONAL REGULATORY STANDARDS
European regulatory bodies, including the European Medicines Agency (EMA) and national authorities, are actively engaged in developing and implementing comprehensive regulations and standards for CSTDs. These standards are designed to ensure the safety, efficacy, and quality of CSTDs across the European market. The harmonization of regulatory requirements promotes consistent and standardized use of CSTDs in diverse healthcare settings.
NIOSH REQUIREMENTS
PRoSeal™ CSTDs meet the NIOSH requirements for closed system transfer devices, mechanically preventing the transfer of environmental contaminants and the escape of hazardous drugs or vapor concentrations. For instance, ProSeal™– ToxiSeal Vial Adaptors, are equipped with an external balloon, in addition to a 0.1 micron sterile filter and activated carbon filters.
PHARMACEUTICAL SAFETY STANDARDS
ProSeal™ CSTDs are FDA ONB & FPA code cleared and compliant with USP<797> and USP<800> standards, demonstrating their commitment to pharmaceutical safety regulations.
TESTS
PROVEN MICROBIAL INGRESS PREVENTION
ProSeal™ CSTDs have been rigorously tested and confirmed to prevent microbial ingress for at least 168 hours after 10 activations, ensuring long lasting effectiveness.
ERGONOMICS
USER-FRIENDLY PUSH/PULL ACTION
ProSeal™ Devices feature a quick and simple Push/Pull action, eliminating the need for repetitive rotation and reducing the risk of RSI-type injuries (Repetitive Strain Injuries).
OPTIMIZATION
COST EFFECTIVE & EFFICIENT
Beyond safety, the design of ProSeal™ CSTDs enables efficient manufacturability, resulting in competitive pricing.
Explore the potential of our
cutting-edge infusion pump solutions.
Contact us for more information or
partnership inquiries and join us in
advancing healthcare together.
BECOME A PARTNTER